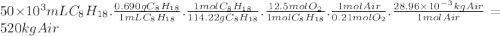Chemistry, 05.12.2019 19:31 mayamcmillan11
Consider stoichiometric combustion of gasoline and air. assume a full tank of gasoline holds 50 l (approximately 13.2 gallons), determine mass of air in kg required to combustion it. you can use isooctane c8h18 to approximate gasoline

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2
You know the right answer?
Consider stoichiometric combustion of gasoline and air. assume a full tank of gasoline holds 50 l (a...
Questions

Mathematics, 27.10.2020 14:00

Social Studies, 27.10.2020 14:00

English, 27.10.2020 14:00


Mathematics, 27.10.2020 14:00

Business, 27.10.2020 14:00

English, 27.10.2020 14:00

Computers and Technology, 27.10.2020 14:00

Mathematics, 27.10.2020 14:00

Business, 27.10.2020 14:00

Biology, 27.10.2020 14:00

Mathematics, 27.10.2020 14:00



Mathematics, 27.10.2020 14:00


Geography, 27.10.2020 14:00

Social Studies, 27.10.2020 14:00

Social Studies, 27.10.2020 14:00

English, 27.10.2020 14:00