
To identify a diatomic gas (x2), a researcher carried out the following experiment: she weighed an empty 5.8-l bulb, then filled it with the gas at 1.00 atm and 21.0 ∘c and weighed it again. the difference in mass was 6.7 g. identify the gas. express your answer as a chemical formula.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
To identify a diatomic gas (x2), a researcher carried out the following experiment: she weighed an...
Questions

History, 14.10.2019 06:30


English, 14.10.2019 06:30


Mathematics, 14.10.2019 06:30




Mathematics, 14.10.2019 06:30



Mathematics, 14.10.2019 06:30




English, 14.10.2019 06:30

Advanced Placement (AP), 14.10.2019 06:30


English, 14.10.2019 06:30

Social Studies, 14.10.2019 06:30

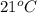 = (21 + 273) K = 294 K, mass = 6.7 g
= (21 + 273) K = 294 K, mass = 6.7 g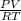
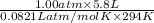
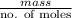

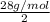
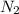 .
.


