
Chemistry, 05.12.2019 20:31 calvinclifton
Asolution is made by mixing exactly 500 ml of 0.156 m naoh with exactly 500 ml of 0.100 m ch3cooh. calculate the equilibrium concentration of the species below. ka of ch3cooh is 1.8 × 10−5
[h+]
× 10 m
enter your answer in scientific notation.
[oh−]
m
[ch3cooh]
× 10 m
enter your answer in scientific notation.
[na+]
m
[ch3coo−]
m

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:50
What is the specific heat of a substance that absorbs 2.5×10^3 joules of heat when a sample of 1.0 ×10^4g of the substance increases in temperature from 10°c to 70°c?
Answers: 2


Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

You know the right answer?
Asolution is made by mixing exactly 500 ml of 0.156 m naoh with exactly 500 ml of 0.100 m ch3cooh. c...
Questions





English, 10.05.2021 08:00

Chemistry, 10.05.2021 08:00

Mathematics, 10.05.2021 08:00

Computers and Technology, 10.05.2021 08:00

History, 10.05.2021 08:00





English, 10.05.2021 08:00



Mathematics, 10.05.2021 08:00



Mathematics, 10.05.2021 08:00

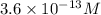 .
.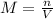
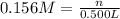
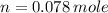
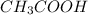 = 500 ml = 0.500L
= 500 ml = 0.500L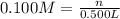
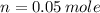
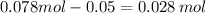
![[H^{+}][OH^{-}]=10^{-14}](/tpl/images/0405/0271/fbb48.png)
![[H^{+}]= \frac {10^{-14}}{[OH^{-}]}](/tpl/images/0405/0271/2c3bc.png)
![[H^{+}]= \frac {10^{-14}}{0.028}=3.6 \times 10^{-14}](/tpl/images/0405/0271/d9745.png)
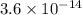 ,
, 

