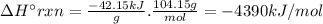Chemistry, 05.12.2019 20:31 leeamation31
Styrene, c8h8, is one of the substances used in the production of synthetic rubber. when styrene burns in oxygen to form carbon dioxide and liquid water under standard-state conditions at 25°c, 42.15 kj are released per gram of styrene. find the standard enthalpy of formation of styrene at 25°c.
(given: ? h°f[co2(g)] = –393.5 kj/mol, ? h°f[h2o(l)] = –285.8 kj/mol, ? h°f[h2o(g)] = –241.8 kj/mol)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1


Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1
You know the right answer?
Styrene, c8h8, is one of the substances used in the production of synthetic rubber. when styrene bur...
Questions







Biology, 20.07.2019 17:00

Biology, 20.07.2019 17:00

Mathematics, 20.07.2019 17:00

History, 20.07.2019 17:00

History, 20.07.2019 17:00



Computers and Technology, 20.07.2019 17:00


Mathematics, 20.07.2019 17:00


Geography, 20.07.2019 17:00