
Silver can be electroplated at the cathode of an electrolysis cell by the half-reaction. ag+(aq)+e−→ag(s) part a silver can be electroplated at the cathode of an electrolysis cell according to the following half-reaction: ag+(aq)+e−→ag(s) what mass of silver plates onto the cathode when a current of 8.5 a flows through the cell for 68 min ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2

Chemistry, 23.06.2019 07:00
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2
You know the right answer?
Silver can be electroplated at the cathode of an electrolysis cell by the half-reaction. ag+(aq)+e−→...
Questions

History, 19.11.2020 07:00



History, 19.11.2020 07:00

Mathematics, 19.11.2020 07:00

Spanish, 19.11.2020 07:00

History, 19.11.2020 07:00


English, 19.11.2020 07:00

History, 19.11.2020 07:00




English, 19.11.2020 07:00

Geography, 19.11.2020 07:00





Chemistry, 19.11.2020 07:00

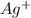 +e− ==> Ag(s)
+e− ==> Ag(s)

