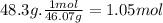Chemistry, 05.12.2019 21:31 rikac72791
How much heat energy is required to convert 48.3 g of solid ethanol at -114.5 degree c to gasesous ethanol at 135.3 degree c? the molar heat of fusion of ethanol is 4.60 kj/mol and its molar heat of vaporization is 38.56 kj/mol. ethanol has a normal melting point of -114.5 degree c and a normal boiling point of 78.4 degree c. the specific heat capacity of liquid ethanol is 2.45 j/g degree c and that of gaseous ethanol is 1.43 j/g degree

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2



Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
How much heat energy is required to convert 48.3 g of solid ethanol at -114.5 degree c to gasesous e...
Questions








Chemistry, 28.01.2020 00:31