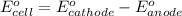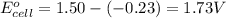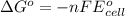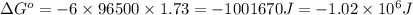Chemistry, 05.12.2019 22:31 angel0203wilcox
Calculate the standard free-energy change for the reaction at 25 ∘ c. 25 ∘c. refer to the list of standard reduction potentials. 2 au 3 + (aq) + 3 ni (s) − ⇀ ↽ − 2 au (s) + 3 ni 2 + (aq) 2au3+(aq)+3ni(s)↽−−⇀2au(s)+3ni2+(aq ) δ g ∘ = δg∘=

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1


Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1

You know the right answer?
Calculate the standard free-energy change for the reaction at 25 ∘ c. 25 ∘c. refer to the list of st...
Questions







Mathematics, 21.07.2019 04:30

Mathematics, 21.07.2019 04:30

Biology, 21.07.2019 04:30

History, 21.07.2019 04:30


Biology, 21.07.2019 04:30

English, 21.07.2019 04:30




Social Studies, 21.07.2019 04:30



Social Studies, 21.07.2019 04:30

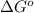 for the given reaction is
for the given reaction is 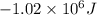
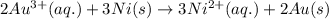
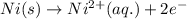 ( × 3)
( × 3)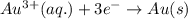 ( × 2)
( × 2)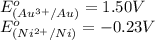
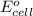 of the reaction, we use the equation:
of the reaction, we use the equation: