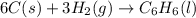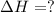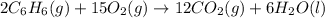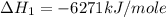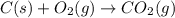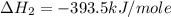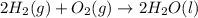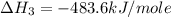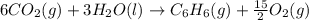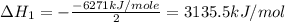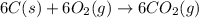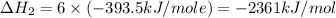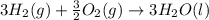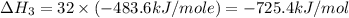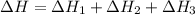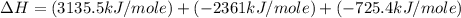Chemistry, 05.12.2019 23:31 carlinryan
Using the equations 2 c₆h₆ (l) + 15 o₂ (g) → 12 co₂ (g) + 6 h₂o (g)∆h° = -6271 kj/mol c (s) + o₂ (g) → co₂ (g) ∆h° = -393.5 kj/mol 2 h₂ (g) + o₂ (g) → 2 h₂o (g) ∆h° = -483.6 kj/mol determine the enthalpy for the reaction 6 c (s) + 3 h₂ (g) → c₆h₆ (l).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3


Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
Using the equations 2 c₆h₆ (l) + 15 o₂ (g) → 12 co₂ (g) + 6 h₂o (g)∆h° = -6271 kj/mol c (s) + o₂ (g)...
Questions

Mathematics, 23.04.2021 20:40

Mathematics, 23.04.2021 20:40




Engineering, 23.04.2021 20:40



Mathematics, 23.04.2021 20:40



Mathematics, 23.04.2021 20:40


Mathematics, 23.04.2021 20:40

Mathematics, 23.04.2021 20:40





Physics, 23.04.2021 20:40

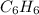 will be,
will be,