
Chemistry, 05.12.2019 23:31 davidleew24
Part b redox titrations are used to determine the amounts of oxidizing and reducing agents in solution. for example, a solution of hydrogen peroxide, h2o2, can be titrated against a solution of potassium permanganate, kmno4. the following equation represents the reaction: 2kmno4(aq)+h2o2(aq)+3h2so4(aq)→3o2( g)+2mnso4(aq)+k2so4(aq)+4h2o(l) a certain amount of hydrogen peroxide was dissolved in 100. ml of water and then titrated with 1.68 m kmno4. what mass of h2o2 was dissolved if the titration required 17.8 ml of the kmno4 solution? express your answer with the appropriate units. view available hint(s) mass of h2o2h 2 o 2 = previous answers incorrect; try again; 5 attempts remaining provide feedback incorrect. incorrect; try again; 5 attempts remaining. no additional feedback.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
Part b redox titrations are used to determine the amounts of oxidizing and reducing agents in soluti...
Questions

History, 01.03.2021 01:00

Mathematics, 01.03.2021 01:00



Mathematics, 01.03.2021 01:00

Biology, 01.03.2021 01:00


Mathematics, 01.03.2021 01:00


Mathematics, 01.03.2021 01:00


Biology, 01.03.2021 01:00

Biology, 01.03.2021 01:00


Mathematics, 01.03.2021 01:00

Physics, 01.03.2021 01:00

Mathematics, 01.03.2021 01:00



Mathematics, 01.03.2021 01:00

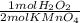 = 0,01495 moles of H₂O₂. As molar mass of H₂O₂ is 34,01g/mol, mass of H₂O₂ was dissolved is:
= 0,01495 moles of H₂O₂. As molar mass of H₂O₂ is 34,01g/mol, mass of H₂O₂ was dissolved is: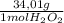 = 0,508g of H₂O₂
= 0,508g of H₂O₂ 

