

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3


Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
You know the right answer?
Ahot air balloon filled with 1.27x10^6l of an ideal gas on a cool morning of 11 degrees c. the air i...
Questions



Mathematics, 25.11.2021 14:00


History, 25.11.2021 14:00

Social Studies, 25.11.2021 14:00

Social Studies, 25.11.2021 14:00









Business, 25.11.2021 14:00


Computers and Technology, 25.11.2021 14:00


Biology, 25.11.2021 14:00

 L
L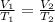
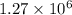 L
L




