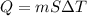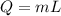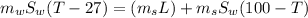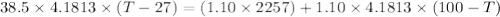Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2


Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

You know the right answer?
If 1.10 g of steam at 100.0 °c condenses into 38.5 g of water, initially at 27.0 °c, in an insulated...
Questions

Biology, 01.09.2019 18:10

Biology, 01.09.2019 18:10

Physics, 01.09.2019 18:10


History, 01.09.2019 18:10

Mathematics, 01.09.2019 18:10

Mathematics, 01.09.2019 18:10

History, 01.09.2019 18:10



Chemistry, 01.09.2019 18:10






Biology, 01.09.2019 18:10



Chemistry, 01.09.2019 18:10