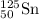Chemistry, 06.12.2019 00:31 ninilizovtskt
An atom of 125sn has a mass of 124.907785 amu. calculate the mass defect (deficit) in amu/atom. use the masses: mass of 1h atom = 1.007825 amu mass of a neutron = 1.008665 amu 1 amu = 931.5 mev give your answer to 4 significant figures and do not use e notation..

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:10
Seawater contains approximately 3.5%nacl by mass and has a density of 1.02 g/ml. what volume of seawater contains 7.5 g of sodium?
Answers: 2

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2
You know the right answer?
An atom of 125sn has a mass of 124.907785 amu. calculate the mass defect (deficit) in amu/atom. use...
Questions






Mathematics, 19.08.2021 02:40

English, 19.08.2021 02:40







Mathematics, 19.08.2021 02:40


Mathematics, 19.08.2021 02:40

Mathematics, 19.08.2021 02:40

Mathematics, 19.08.2021 02:50

Mathematics, 19.08.2021 02:50

Mathematics, 19.08.2021 02:50