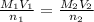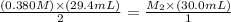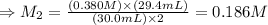Chemistry, 06.12.2019 02:31 NathanFrase6770
The amount of i − 3 ( aq ) in a solution can be determined by titration with a solution containing a known concentration of s 2 o 2 − 3 ( aq ) (thiosulfate ion). the determination is based on the net ionic equation 2 s 2 o 2 − 3 ( aq ) + i − 3 ( aq ) ⟶ s 4 o 2 − 6 ( aq ) + 3 i − ( aq ) given that it requires 29.4 ml of 0.380 m na 2 s 2 o 3 ( aq ) to titrate a 30.0 ml sample of i − 3 ( aq ) , calculate the molarity of i − 3 ( aq ) in the solution.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1
You know the right answer?
The amount of i − 3 ( aq ) in a solution can be determined by titration with a solution containing a...
Questions

Mathematics, 11.08.2021 15:50

Biology, 11.08.2021 15:50

Mathematics, 11.08.2021 15:50

Health, 11.08.2021 15:50

Computers and Technology, 11.08.2021 15:50

English, 11.08.2021 15:50

Mathematics, 11.08.2021 15:50



Mathematics, 11.08.2021 15:50


Mathematics, 11.08.2021 15:50



Biology, 11.08.2021 15:50

English, 11.08.2021 15:50

History, 11.08.2021 15:50

Mathematics, 11.08.2021 15:50

English, 11.08.2021 15:50

French, 11.08.2021 15:50