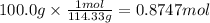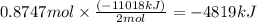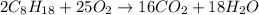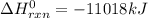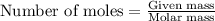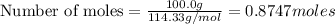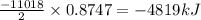Chemistry, 06.12.2019 02:31 autumnguidry7628
Using the following equation for the combustion of octane, calculate the heat associated with the combustion of 100.0 g of octane assuming complete combustion. the molar mass of octane is 114.33 g/mole. the molar mass of oxygen is 31.9988 g/mole.
2 c8h18 + 25 o2 → 16 co2 + 18 h2o δh°rxn = -11018 kj
a) -535.4 kj
b) -4819 kj
c) -602.3 kj
d) -385.5 kj
e) -11018 kj

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 03:30
Ineed pls urgent 1-20 in order and fully detail step my step.
Answers: 1
You know the right answer?
Using the following equation for the combustion of octane, calculate the heat associated with the co...
Questions



Mathematics, 24.03.2020 23:07

English, 24.03.2020 23:07

Mathematics, 24.03.2020 23:07



Mathematics, 24.03.2020 23:07

Mathematics, 24.03.2020 23:07

History, 24.03.2020 23:07



Computers and Technology, 24.03.2020 23:07

Biology, 24.03.2020 23:07


Mathematics, 24.03.2020 23:07