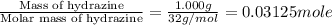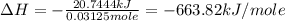Chemistry, 06.12.2019 03:31 isabeltorres5
A1.000 gram sample of the rocket fuel hydrazine (n2h4) is burned in a bomb calorimeter. the temperature rises from 24.62°c to 28.16°c. the heat capacity of the calorimeter (including the water) is 5860 j/°c. calculate the molar heat of combustion of hydrazine, in kj/mole.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
A1.000 gram sample of the rocket fuel hydrazine (n2h4) is burned in a bomb calorimeter. the temperat...
Questions

Biology, 20.10.2019 00:50

Mathematics, 20.10.2019 00:50

English, 20.10.2019 00:50


Mathematics, 20.10.2019 00:50

Computers and Technology, 20.10.2019 00:50


History, 20.10.2019 00:50

Social Studies, 20.10.2019 00:50


Mathematics, 20.10.2019 00:50


Spanish, 20.10.2019 00:50



History, 20.10.2019 00:50


Biology, 20.10.2019 00:50


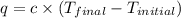
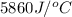
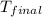 = final temperature =
= final temperature = 
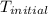 = initial temperature =
= initial temperature = 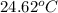
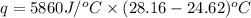
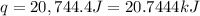
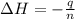
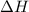 = enthalpy change = ?
= enthalpy change = ?