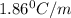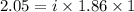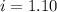Chemistry, 06.12.2019 03:31 Unkn0wn3815
You are given a 1.00 molal solution of an unknown solute dissolved in water and told that you must determine if the solute is a strong electrolyte, a weak electrolyte, or a nonelectrolyte. on testing, you find that the freezing point of the solution is -2.05 degrees celsius. is this enough information to answer the question? the molal freezing point depression constant for water is 1.86 °c/m. o no, this is not enough information o yes, the solute is a nonelectrolyte yes, the solute is a weak electrolyte yes, the solute is a strong electrolyte

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
You are given a 1.00 molal solution of an unknown solute dissolved in water and told that you must d...
Questions





Biology, 21.01.2020 17:31









Social Studies, 21.01.2020 17:31

Mathematics, 21.01.2020 17:31

English, 21.01.2020 17:31

Arts, 21.01.2020 17:31


English, 21.01.2020 17:31

Mathematics, 21.01.2020 17:31

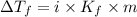
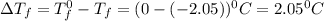 = Depression in freezing point
= Depression in freezing point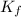 = freezing point constant =
= freezing point constant =