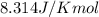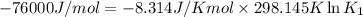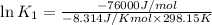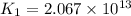Chemistry, 06.12.2019 04:31 emilybomar7466
Consider the reaction 4 hcl(g) + o2(g) =2 h2o(g) + 2 cl2(g) using the standard thermodynamic data in the tables linked above, calculate the equilibrium constant for this reaction at 298.15k.
delta g (kj/mol)
hcl=-95.3
o2=0
h2o=-228.6
cl2=0

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1

Chemistry, 23.06.2019 08:10
An experiment is conducted to see if cats preferred skim milk or 2% milk. a cup of skim milkwas put out for 5 kittens and then measured how much the kittens drank over the course of aday. following a cup of 2% milk was purout for the skittens and then masured how much thekittens drank over the course of a day. the same kittens were used and the milk was served atthe same temperature. it was discovered that the cats liked the 2% milk more than the skimmilk. what is the dependent variable in this experiment?
Answers: 1

Chemistry, 23.06.2019 09:10
Complete the following radioactive decay problem. tan+on-? c+th
Answers: 1

Chemistry, 23.06.2019 09:40
Write balanced nuclear equations for the formation of five elements whose atomic number is between helium (2) and iron (26):
Answers: 1
You know the right answer?
Consider the reaction 4 hcl(g) + o2(g) =2 h2o(g) + 2 cl2(g) using the standard thermodynamic data in...
Questions

English, 16.01.2020 07:31


Advanced Placement (AP), 16.01.2020 07:31

Spanish, 16.01.2020 07:31


Mathematics, 16.01.2020 07:31


Mathematics, 16.01.2020 07:31


Health, 16.01.2020 07:31


Mathematics, 16.01.2020 07:31

Social Studies, 16.01.2020 07:31


Biology, 16.01.2020 07:31





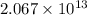 .
.![\Delta G^o_{rxn}=\sum [n\times \Delta G^o_f(product)]-\sum [n\times \Delta G^o_f(reactant)]](/tpl/images/0405/8552/b00b4.png)
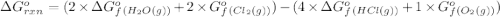
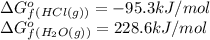
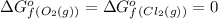 (pure element)
(pure element)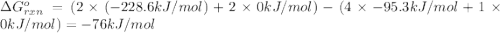
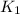 (at 25°C) for given value of Gibbs free energy, we use the relation:
(at 25°C) for given value of Gibbs free energy, we use the relation: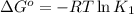
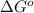 = Gibbs free energy = -76 kJ/mol = -76000 J/mol
= Gibbs free energy = -76 kJ/mol = -76000 J/mol