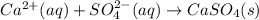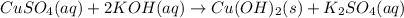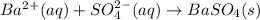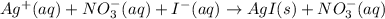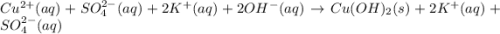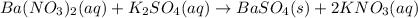Chemistry, 06.12.2019 04:31 bikkiecuanas13
Using what you have learned about the three forms of writing chemical equations, classify each of the presented equations as either a molecular equation, a complete ionic equation, or a net ionic equation. drag the appropriate equations to their respective bins.
a)ca2+(aq)+so42−(aq)→caso4(s)b)cuso 4(aq)+2koh(aq)→cu(oh)2(s)+k2so4(aq) c)ba2+(aq)+so42−(aq)→baso4(s)
d)ag+(aq)+no3−(aq)+i−(aq)→agi(s)+no 3−(aq)e)cu2+(aq)+so42−(aq)+2k+(aq)+ 2oh−(aq)→cu(oh)2(s)+2k+(aq)+so42−(a q)f)(bano3)2(aq)+k2so4(aq)→baso4(s) +2kno3(aq)molecular equationcomplete ionic equation

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1
You know the right answer?
Using what you have learned about the three forms of writing chemical equations, classify each of th...
Questions

Mathematics, 22.09.2019 21:30


English, 22.09.2019 21:30

Social Studies, 22.09.2019 21:30

Mathematics, 22.09.2019 21:30

English, 22.09.2019 21:30

Computers and Technology, 22.09.2019 21:30

Mathematics, 22.09.2019 21:30



Mathematics, 22.09.2019 21:30



English, 22.09.2019 21:30




Mathematics, 22.09.2019 21:30