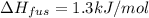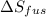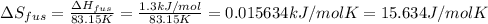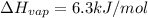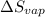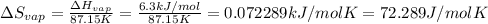Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

You know the right answer?
The molar heats of fusion and vaporization of argon are 1.3 kj/mol and 6.3 kj/mol respectively, and...
Questions


Mathematics, 10.02.2021 23:40





Mathematics, 10.02.2021 23:40


Mathematics, 10.02.2021 23:40


Mathematics, 10.02.2021 23:40

Mathematics, 10.02.2021 23:40

English, 10.02.2021 23:40


Mathematics, 10.02.2021 23:40

History, 10.02.2021 23:40

Physics, 10.02.2021 23:40


Mathematics, 10.02.2021 23:40

Mathematics, 10.02.2021 23:40