
Chemistry, 06.12.2019 05:31 smkw04p3ao0n
The equilibrium constant, kc, for the following reaction is 5.10×10-6 at 548 k. nh4cl(s) nh3(g) + hcl(g) calculate the equilibrium concentration of hcl when 0.564 moles of nh4cl(s) are introduced into a 1.00 l vessel at 548 k.[hcl] = m

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3
You know the right answer?
The equilibrium constant, kc, for the following reaction is 5.10×10-6 at 548 k. nh4cl(s) nh3(g) + hc...
Questions

Mathematics, 09.12.2020 23:20




History, 09.12.2020 23:20




English, 09.12.2020 23:20

Mathematics, 09.12.2020 23:20




Mathematics, 09.12.2020 23:20

Mathematics, 09.12.2020 23:20


Mathematics, 09.12.2020 23:20


Biology, 09.12.2020 23:20

Mathematics, 09.12.2020 23:20

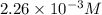
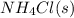 = 0.564 moles
= 0.564 moles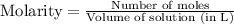
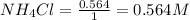
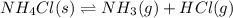
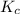 for above equation follows:
for above equation follows:![K_c=[NH_3][HCl]](/tpl/images/0405/9498/72be1.png)
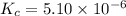
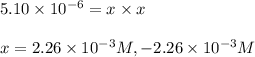
![[HCl]=2.26\times 10^{-3}M](/tpl/images/0405/9498/283fd.png)


