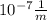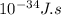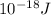Calculate the energy required to excite the hydrogen electron from n = 1 to level n = 2. also calculate the wavelength of light that must be absorbed by a hydrogen atom in its ground state to reach this excited state. what kind of electromagnetic radiation is used?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1


You know the right answer?
Calculate the energy required to excite the hydrogen electron from n = 1 to level n = 2. also calcul...
Questions

Chemistry, 05.05.2021 23:00

German, 05.05.2021 23:00

Social Studies, 05.05.2021 23:00



Chemistry, 05.05.2021 23:00

Mathematics, 05.05.2021 23:00




English, 05.05.2021 23:00


Mathematics, 05.05.2021 23:00


Chemistry, 05.05.2021 23:00


Health, 05.05.2021 23:00



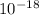 J, the wavelength is 1.215×
J, the wavelength is 1.215×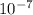 m, and the kind of electromagnetic radiation is the ultraviolet radiation.
m, and the kind of electromagnetic radiation is the ultraviolet radiation.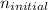 = 1, and
= 1, and 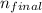 = 2. Then, we use Rydberg's equation to calculate the wavelength of the light absorbed by the atom during the transition.
= 2. Then, we use Rydberg's equation to calculate the wavelength of the light absorbed by the atom during the transition.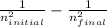 )
)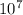
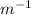
 ) = 1.0974×
) = 1.0974× = (-) 0.8228*10^{7}
= (-) 0.8228*10^{7}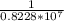 = 1.215*
= 1.215*