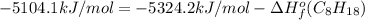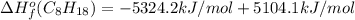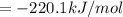Chemistry, 06.12.2019 05:31 breannamiller0822
Or a particular isomer of c 8 h 18 , the combustion reaction produces 5104.1 kj of heat per mole of c 8 h 18 ( g ) consumed, under standard conditions. c 8 h 18 ( g ) + 25 2 o 2 ( g ) ⟶ 8 co 2 ( g ) + 9 h 2 o ( g ) δ h ∘ rxn = − 5104.1 kj / mol what is the standard enthalpy of formation of this isomer of c 8 h 18 ( g ) ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

You know the right answer?
Or a particular isomer of c 8 h 18 , the combustion reaction produces 5104.1 kj of heat per mole of...
Questions

Social Studies, 18.02.2021 17:30

Mathematics, 18.02.2021 17:30



Business, 18.02.2021 17:30


Mathematics, 18.02.2021 17:30


Physics, 18.02.2021 17:30






Chemistry, 18.02.2021 17:30

Mathematics, 18.02.2021 17:30

Mathematics, 18.02.2021 17:30


Chemistry, 18.02.2021 17:30

Mathematics, 18.02.2021 17:30

 is -220.1 kJ/mol.
is -220.1 kJ/mol.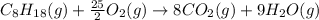

![\Delta H^{o}_{rxn}=[8\Delta H^{o}_{f}(CO_{2}) +9\Delta H^{o}_{f}(H_{2}O)]-[\Delta H^{o}_{f}(C_{8}H_{18})+ \frac{25}{2}\Delta H^{o}_{f}(O_{2})]](/tpl/images/0405/9197/1d3a2.png)
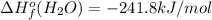
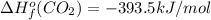

![-5104.1kJ/mol=[8(-393.5)+9(-241.8)kJ/mol]-[\Delta H^{o}_{f}(C_{8}H_{18})+ \frac{25}{2}(0)kJ/mol]](/tpl/images/0405/9197/edc02.png)