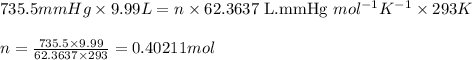Oxygen gas can be prepared by heating potassium chlorate according to the following equation: 2kclo3(s)arrow. gif2kcl(s) + 3o2(g)the product gas, o2, is collected over water at a temperature of 20 °c and a pressure of 748 mm hg. if the wet o2 gas formed occupies a volume of 9.49 l, the number of moles of kclo3 reacted was ? mol. the vapor pressure of water is 17.5 mm hg at 20 °c. oxygen gas can be prepared by heating potassium chlorate according to the following equation: 2kclo3(s)arrow. gif2kcl(s) + 3o2(g)the product gas, o2, is collected over water at a temperature of 20 °c and a pressure of 753 mm hg. if the wet o2 gas formed occupies a volume of 9.99 l, the number of grams of o2 formed is ? g. the vapor pressure of water is17.5 mm hg at 20 °c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 05:40
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1
You know the right answer?
Oxygen gas can be prepared by heating potassium chlorate according to the following equation: 2kclo3...
Questions

Social Studies, 04.03.2021 20:30



Biology, 04.03.2021 20:30

Mathematics, 04.03.2021 20:30



Mathematics, 04.03.2021 20:30

Mathematics, 04.03.2021 20:30


English, 04.03.2021 20:30





Mathematics, 04.03.2021 20:30


Spanish, 04.03.2021 20:30


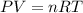
![20^oC=[20+273]K=293K](/tpl/images/0406/0694/3b5d4.png)
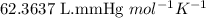
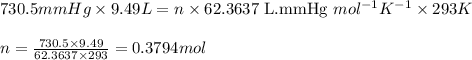
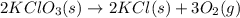
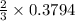 moles of potassium chlorate undergoes reaction.
moles of potassium chlorate undergoes reaction.