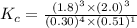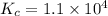Chemistry, 06.12.2019 06:31 btcastongia
Part a what is the numerical value of kc for the following reaction if the equilibrium mixture contains 0.51 m c3h6o, 0.30 m o2, 1.8 m co2, and 2.0 m h2o? c3h6o(g)+4o2(g)⇌3co2(g)+3h2o(g)
a) 2.4 × 101b) 1.1 × 104c) 8.9 × 10-5d) 4.3 × 10-2

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

You know the right answer?
Part a what is the numerical value of kc for the following reaction if the equilibrium mixture conta...
Questions

Physics, 26.12.2020 06:50

Health, 26.12.2020 06:50



Business, 26.12.2020 07:10

Computers and Technology, 26.12.2020 07:10

Mathematics, 26.12.2020 07:10

Social Studies, 26.12.2020 07:10


Mathematics, 26.12.2020 07:10



Mathematics, 26.12.2020 07:10

Social Studies, 26.12.2020 07:10

History, 26.12.2020 07:10

History, 26.12.2020 07:10

Physics, 26.12.2020 07:10

Mathematics, 26.12.2020 07:10

Computers and Technology, 26.12.2020 07:20

English, 26.12.2020 07:20


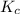 .
.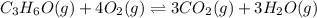
![K_c=\frac{[CO_2]^3\times [H_2O]^3}{[O_2]^4\times [C_3H_6O]^1}](/tpl/images/0406/0681/8cf44.png)