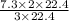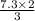Chemistry, 06.12.2019 06:31 KariSupreme
Balance the equation and determine how many liter of nitrogen are required to react with 7.3 l of hydrogen to produce ammonia (nitrogen trihydride)?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
Balance the equation and determine how many liter of nitrogen are required to react with 7.3 l of hy...
Questions

English, 01.09.2020 08:01

Mathematics, 01.09.2020 08:01


Computers and Technology, 01.09.2020 08:01



Geography, 01.09.2020 08:01

Mathematics, 01.09.2020 08:01


History, 01.09.2020 08:01

Social Studies, 01.09.2020 08:01

Mathematics, 01.09.2020 08:01

Mathematics, 01.09.2020 08:01

Chemistry, 01.09.2020 08:01

Social Studies, 01.09.2020 08:01

Mathematics, 01.09.2020 08:01

Social Studies, 01.09.2020 08:01


Geography, 01.09.2020 08:01

Mathematics, 01.09.2020 08:01