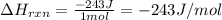4). one mole of monoclinic sulfur at 25c was placed in a constant-pressure calorimeter whose heat capacity (c) was 1620 j/k. the temperature of the calorimeter increased by 0.150 co when the sulfur changed from the monoclinic to the orthorhombic form. calculate the enthalpy change for the process s(monoclinic) s(orthorhombic).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2

Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
You know the right answer?
4). one mole of monoclinic sulfur at 25c was placed in a constant-pressure calorimeter whose heat c...
Questions



Chemistry, 20.04.2020 07:58

Social Studies, 20.04.2020 07:58

Mathematics, 20.04.2020 07:58

Mathematics, 20.04.2020 07:58


Mathematics, 20.04.2020 07:58

Business, 20.04.2020 07:58


Mathematics, 20.04.2020 07:58

Arts, 20.04.2020 07:58

History, 20.04.2020 07:58

Mathematics, 20.04.2020 07:58

Mathematics, 20.04.2020 07:58

Mathematics, 20.04.2020 07:58


Mathematics, 20.04.2020 07:58


Chemistry, 20.04.2020 07:58

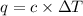
 = change in temperature =
= change in temperature = 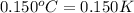 (Change remains same)
(Change remains same)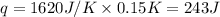
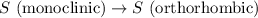
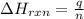
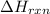 = enthalpy change of the reaction
= enthalpy change of the reaction