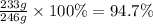Chemistry, 06.12.2019 19:31 yungnoaweo4209
The solubility of potassium nitrate is 13 g per 100. ml of water at 0 oc and 246 g per 100. ml of water at 100.oc. a quantity of 246 g of potassium nitrate is dissolved in 100. ml of water at 100. oc and the solution is cooled to 0 oc. what percentage by mass of the kno3 crystallizes at 0oc?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2
You know the right answer?
The solubility of potassium nitrate is 13 g per 100. ml of water at 0 oc and 246 g per 100. ml of wa...
Questions



History, 13.01.2021 19:00


Mathematics, 13.01.2021 19:00

Mathematics, 13.01.2021 19:00



Physics, 13.01.2021 19:00




Mathematics, 13.01.2021 19:10


Health, 13.01.2021 19:10





Mathematics, 13.01.2021 19:10