
Chemistry, 06.12.2019 19:31 anthonyvargas8780
Astandard nitric acid solution is prepared using 0.425 g of sodium carbonate, na2co3. the balanced equation is:
2 hno3 (aq) + na2co3 (s) ➝ 2 nano3 (aq) + h2o (l) + co2 (g)
referring to example exercise 2, provide in three significant figures:
a) # moles of na2co3
b) # moles of hno3
c) molarity of hno3 if 33.25 ml are required to reach a permanent endpoint.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 23.06.2019 13:10
When can a hypothesis be elevated to the status of a theory? a) when it is validated by an experiment b) when data gathered from an experiment precisely fits predictions c) when it can be proved to be true d) when it meets the test of repeated experimentation
Answers: 2

Chemistry, 23.06.2019 16:30
Which temperature scale measures water boiling at 100 degrees?
Answers: 2
You know the right answer?
Astandard nitric acid solution is prepared using 0.425 g of sodium carbonate, na2co3. the balanced e...
Questions



English, 01.01.2022 18:40



Mathematics, 01.01.2022 18:50

English, 01.01.2022 18:50

Mathematics, 01.01.2022 18:50

Spanish, 01.01.2022 18:50



Mathematics, 01.01.2022 18:50



Health, 01.01.2022 18:50

English, 01.01.2022 18:50

Mathematics, 01.01.2022 18:50

Mathematics, 01.01.2022 18:50


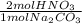 = 8.02x10⁻³ mol HNO₃
= 8.02x10⁻³ mol HNO₃


