
Chemistry, 06.12.2019 19:31 alyssalefeber
The molal boiling point elevation constant kb= 2.13 ℃kgmo-for a certain substance x, when 12. g of urea are dissolved in 100. g of x, the solution boils at 126.3 °c. calculate the boiling point of pure x. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2
You know the right answer?
The molal boiling point elevation constant kb= 2.13 ℃kgmo-for a certain substance x, when 12. g of u...
Questions

Biology, 27.04.2021 15:30

Chemistry, 27.04.2021 15:30





Computers and Technology, 27.04.2021 15:30


Mathematics, 27.04.2021 15:30

Mathematics, 27.04.2021 15:30

Mathematics, 27.04.2021 15:30

Computers and Technology, 27.04.2021 15:30



Mathematics, 27.04.2021 15:30




Mathematics, 27.04.2021 15:30

Social Studies, 27.04.2021 15:30

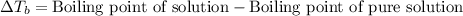
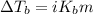
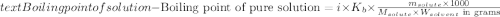
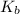 = molal boiling point elevation constant = 2.13°C/m
= molal boiling point elevation constant = 2.13°C/m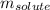 = Given mass of solute (urea) = 12. g
= Given mass of solute (urea) = 12. g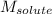 = Molar mass of solute (urea) = 60 g/mol
= Molar mass of solute (urea) = 60 g/mol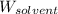 = Mass of solvent (X) = 100. g
= Mass of solvent (X) = 100. g


