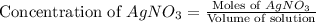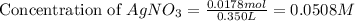Chemistry, 06.12.2019 19:31 orlando19882000
Suppose a 2.95 g of potassium iodide is dissolved in 350. ml of a 62.0 m m aqueous solution of silver nitrate. calculate the final molarity of iodide anion in the solution. you can assume the volume of the solution doesn't change when the potassium iodide is dissolved in it. be sure your answer has the correct number of significant digits

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1


Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
Suppose a 2.95 g of potassium iodide is dissolved in 350. ml of a 62.0 m m aqueous solution of silve...
Questions

Physics, 24.07.2019 00:30



Spanish, 24.07.2019 00:30

Health, 24.07.2019 00:30


English, 24.07.2019 00:30

English, 24.07.2019 00:30

Computers and Technology, 24.07.2019 00:30

Geography, 24.07.2019 00:30




Social Studies, 24.07.2019 00:30



History, 24.07.2019 00:30

History, 24.07.2019 00:30

Health, 24.07.2019 00:30

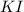 and
and 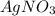 .
.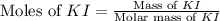
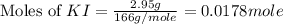
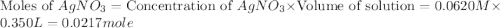
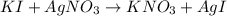
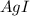
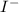 anion = Moles of
anion = Moles of 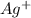 cation = 0.0178 moles
cation = 0.0178 moles