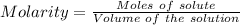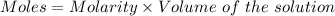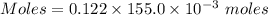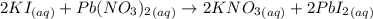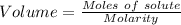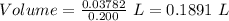Potassium iodide reacts with lead(ii) nitrate in the following precipitation reaction:
2ki(a...

Chemistry, 06.12.2019 20:31 milkshakegrande101
Potassium iodide reacts with lead(ii) nitrate in the following precipitation reaction:
2ki(aq)+pb(no3)2(aq)→2kno3(aq)+pbi2 (s)
what minimum volume of 0.200 m potassium iodide solution is required to completely precipitate all of the lead in 155.0 ml of a 0.122 m lead(ii) nitrate solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2
You know the right answer?
Questions

Mathematics, 30.07.2019 11:00



History, 30.07.2019 11:00

Mathematics, 30.07.2019 11:00




Biology, 30.07.2019 11:00



Physics, 30.07.2019 11:00

History, 30.07.2019 11:00


Mathematics, 30.07.2019 11:10

Mathematics, 30.07.2019 11:10

English, 30.07.2019 11:10