
Only answer if you know for sure
where are the anion and cation written in an ionic compound?
a) the ion with the largest subscript in the formula is written first in the name of an ionic compound
b) the cation is written before the anion in the name and formula of an ionic compound
c) the anion is written before the cation in the name and formula of an ionic compound
d) the ion, cation, or anion with the greatest charge is written first in the name and the formula of an ionic compound

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
Only answer if you know for sure
where are the anion and cation written in an ionic compound?...
where are the anion and cation written in an ionic compound?...
Questions

Mathematics, 16.10.2020 16:01

Mathematics, 16.10.2020 16:01


History, 16.10.2020 16:01


Social Studies, 16.10.2020 16:01


Business, 16.10.2020 16:01

Mathematics, 16.10.2020 16:01


History, 16.10.2020 16:01








History, 16.10.2020 16:01

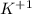 is called the potassium ion but simply 'K' refers potassium atom. Anion is called by the name of the element, removing the end part and adding "ide". For example,
is called the potassium ion but simply 'K' refers potassium atom. Anion is called by the name of the element, removing the end part and adding "ide". For example,  is called fluorine, an element called fluorine. 'ine' was replaced with an 'ide'. To name the compound, the cation and anion names are added together. For example, NaF (sodium fluoride).
is called fluorine, an element called fluorine. 'ine' was replaced with an 'ide'. To name the compound, the cation and anion names are added together. For example, NaF (sodium fluoride).


