

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1
You know the right answer?
In order to produce sp3d hybrid orbitals, s atomic orbital(s), p atomic orbital(s), and d atomic orb...
Questions








History, 25.03.2021 22:30


Computers and Technology, 25.03.2021 22:30

Mathematics, 25.03.2021 22:30

History, 25.03.2021 22:30


Chemistry, 25.03.2021 22:30

Mathematics, 25.03.2021 22:30

Mathematics, 25.03.2021 22:30

Mathematics, 25.03.2021 22:30

Health, 25.03.2021 22:30

Mathematics, 25.03.2021 22:30

Mathematics, 25.03.2021 22:30

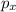 ,
, 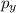 and
and 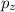 are half filled.
are half filled.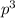 d hybrid orbitals, 1 s, 3 p and 1 d orbitals of approximately the same energy intermix are used to create 5 identical/degenerate hybrid orbitals. These orbitals are arranged to form a trigonal bipyramidal symmetry. Three orbitals are arranged in a trigonal plane and the other two orbitals are arranged up/down the trigonal plane at right angles.
d hybrid orbitals, 1 s, 3 p and 1 d orbitals of approximately the same energy intermix are used to create 5 identical/degenerate hybrid orbitals. These orbitals are arranged to form a trigonal bipyramidal symmetry. Three orbitals are arranged in a trigonal plane and the other two orbitals are arranged up/down the trigonal plane at right angles. 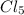 as an example, its formation requires five unpaired electron, the phosphorus atom was excited and one electron from the 3s filled one of the vacant 3d orbitals. Thus intermixing the orbitals created the s
as an example, its formation requires five unpaired electron, the phosphorus atom was excited and one electron from the 3s filled one of the vacant 3d orbitals. Thus intermixing the orbitals created the s

