Chemistry, 07.12.2019 01:31 pharadorvil04
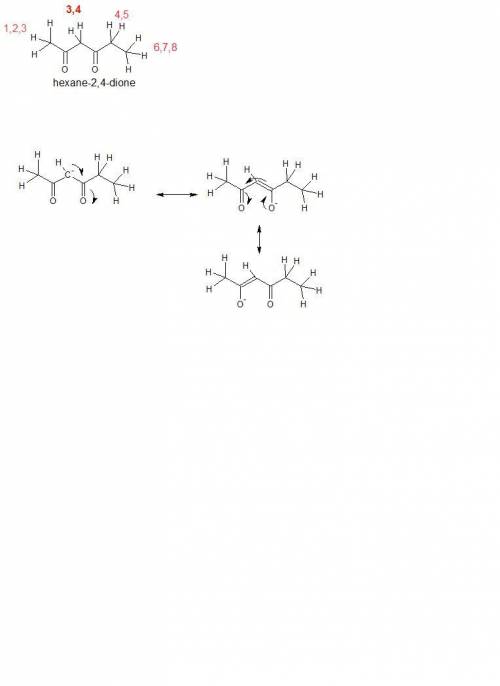
Using resonance, select the most acidic protons in the hexane-2,4-dione molecule. the most acidic proton is the one that, upon removal, will yield the most stable conjugate base.
check all that apply.
[hexane-2,4-dione molecule with protons numbered as follows according to iupac nomenclature: protons on c1 are numbered 1 through 3, protons on c3 are numbered 4 and 5, protons on c5 are numbered 6 and 7, protons on c6 are numbered 8 through 10.]
check all that apply.
protons 1, 2, or 3
protons 4 or 5
protons 6 or 7
protons 8, 9, or 10

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How does decreasing the gas volume affect the pressure of a gas?
Answers: 1

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3
You know the right answer?
Using resonance, select the most acidic protons in the hexane-2,4-dione molecule. the most acidic pr...
Questions


Social Studies, 09.04.2020 23:33

Mathematics, 09.04.2020 23:33

History, 09.04.2020 23:33

History, 09.04.2020 23:33

History, 09.04.2020 23:33

Mathematics, 09.04.2020 23:33




Mathematics, 09.04.2020 23:33




Mathematics, 09.04.2020 23:33





Mathematics, 09.04.2020 23:33