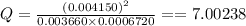Chemistry, 07.12.2019 02:31 aprilkenedy12
Consider this equilibrium reaction at 400 k. br2(g)+cl2(g)↽−−⇀2brcl(g)kc=7.0 br 2 ( g ) + cl 2 ( g ) ↽ − − ⇀ 2 brcl ( g ) k c = 7.0 if the composition of the reaction mixture at 400 k is [brcl]=0.004150 [ brcl ] = 0.004150 m, [br2]=0.003660 [ br 2 ] = 0.003660 m, and [cl2]=0.0006720 [ cl 2 ] = 0.0006720 m, what is the reaction quotient, q ?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2
You know the right answer?
Consider this equilibrium reaction at 400 k. br2(g)+cl2(g)↽−−⇀2brcl(g)kc=7.0 br 2 ( g ) + cl 2 ( g )...
Questions

English, 01.06.2021 22:50


Mathematics, 01.06.2021 22:50


Mathematics, 01.06.2021 22:50

Mathematics, 01.06.2021 22:50






Mathematics, 01.06.2021 22:50

Biology, 01.06.2021 22:50





History, 01.06.2021 22:50

English, 01.06.2021 22:50

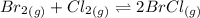
![Q=\frac{[BrCl]^2}{[Br_2][Cl_2]}](/tpl/images/0407/4151/a2d2e.png)
![[BrCl]=0.004150\ M](/tpl/images/0407/4151/33a25.png)
![[Br_2]=0.003660\ M](/tpl/images/0407/4151/93561.png)
![[Cl_2}]=0.0006720\ M](/tpl/images/0407/4151/b53a5.png)