Chemistry, 07.12.2019 02:31 queenkimm26
Consider these hypothetical chemicalreactions:
{\rm a \rightleftharpoons b}, \quad\delta g = 13.2 kj/mol
{\rm b \rightleftharpoons c}, \quad\delta g = -28.9 kj/mol
{\rm c \rightleftharpoons d},\quad \delta g = 5.80 kj/mol
what is the free energy, delta g, for the overall reaction, \rm a \rightleftharpoons d ?
express your answer numerically inkilojoules per mole.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1
You know the right answer?
Consider these hypothetical chemicalreactions:
{\rm a \rightleftharpoons b}, \quad\delta g =...
{\rm a \rightleftharpoons b}, \quad\delta g =...
Questions






History, 24.02.2021 17:10



Mathematics, 24.02.2021 17:10

Mathematics, 24.02.2021 17:10



Mathematics, 24.02.2021 17:10



Business, 24.02.2021 17:10


English, 24.02.2021 17:10


Mathematics, 24.02.2021 17:10

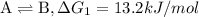 ...[1]
...[1]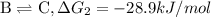 ...[2]
...[2]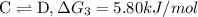 ...[3]
...[3] ...[4]
...[4]