
Chemistry, 07.12.2019 02:31 jdvazquez18p7a7vs
Consider an electrochemical cell constructed from the following half cells, linked by a kcl salt bridge.• a fe electrode in 1.0 m fecl2 solution• a sn electrode in 1.0 m sn(no3)2 solutionwhen the cell is running spontaneously, which choice includes only true statements and no false ones? question 1 options: a. the tin electrode loses mass and the tin electrode is the cathode. b. the tin electrode gains mass and the tin electrode is the cathode. c. the iron electrode gains mass and the iron electrode is the anode. d. the iron electrode loses mass and the iron electrode is the cathode.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1


You know the right answer?
Consider an electrochemical cell constructed from the following half cells, linked by a kcl salt bri...
Questions

English, 26.02.2020 01:02



History, 26.02.2020 01:02


Mathematics, 26.02.2020 01:02


Mathematics, 26.02.2020 01:02










Mathematics, 26.02.2020 01:02

Biology, 26.02.2020 01:02

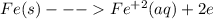
 is higher than tin(ii) ion,
is higher than tin(ii) ion, 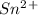 in the electrochemical series, therefore, tin (ii) iron is preferentially discharged to iron(ii) ion in aqueous solutions.Also, when oxidation occurs at the anode and reduction occurs at the cathode, in the Galvanic cell constructed from the following half cells which is linked by a
in the electrochemical series, therefore, tin (ii) iron is preferentially discharged to iron(ii) ion in aqueous solutions.Also, when oxidation occurs at the anode and reduction occurs at the cathode, in the Galvanic cell constructed from the following half cells which is linked by a 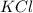 salt bridge:So, an Fe electrode in 1.0 M
salt bridge:So, an Fe electrode in 1.0 M 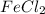 solution and a
solution and a 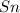 electrode in 1.0 M
electrode in 1.0 M 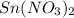 solution, the Fe electrode serves as the anode, while the Sn electrode serves as the cathode.The
solution, the Fe electrode serves as the anode, while the Sn electrode serves as the cathode.The 

