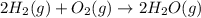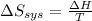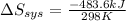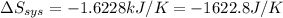Consider the following reaction at 298 k:
2h2(g) + o2(g) --> 2h2o(g) deltah= -483.6...

Chemistry, 07.12.2019 02:31 helloitschump0vfdz
Consider the following reaction at 298 k:
2h2(g) + o2(g) --> 2h2o(g) deltah= -483.6 kj
calculate the following quantities.
delta s sys=
delta s surr=
delta s univ=

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
Questions

Mathematics, 29.06.2019 19:00


Physics, 29.06.2019 19:10


Chemistry, 29.06.2019 19:10

Mathematics, 29.06.2019 19:10


English, 29.06.2019 19:10


History, 29.06.2019 19:10

Mathematics, 29.06.2019 19:10

History, 29.06.2019 19:10


History, 29.06.2019 19:10




Mathematics, 29.06.2019 19:10

English, 29.06.2019 19:10

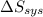 = -1622.8 J/K
= -1622.8 J/K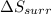 = -94.6 J/K
= -94.6 J/K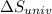 = 0 J/K
= 0 J/K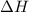 = -483.6 kJ
= -483.6 kJ