
Chemistry, 07.12.2019 03:31 cbender30p860we
Calculate the change in entropy of the system when 10g of ice at -10c is converted into water vapor at 115c and at a constant pressure of 1bar. the constant pressure molar heat capacity of h2o(s) and h2o(l) is 75.291j/kmol and that of h2o(g) is 33.58j/kmo

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3
You know the right answer?
Calculate the change in entropy of the system when 10g of ice at -10c is converted into water vapor...
Questions






Mathematics, 07.01.2021 17:40

Social Studies, 07.01.2021 17:40

Mathematics, 07.01.2021 17:40


English, 07.01.2021 17:40


Mathematics, 07.01.2021 17:40


Mathematics, 07.01.2021 17:40

Geography, 07.01.2021 17:40

Mathematics, 07.01.2021 17:40



Chemistry, 07.01.2021 17:40

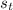 = Δ
= Δ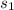 +Δ
+Δ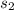 +Δ
+Δ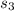 +Δ
+Δ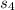
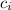 *
*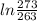
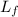 /273
/273 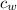 *
*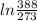
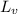 /388
/388 

