
Chemistry, 07.12.2019 03:31 brainBoy480
Arock contains 0.275 mg of lead-206 for each milligram of uranium-238. the for the decay of uranium-238 to lead-206 is 4.5 x 10 9 yr. the rock was formed yr ago.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1


Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
Arock contains 0.275 mg of lead-206 for each milligram of uranium-238. the for the decay of uranium-...
Questions


English, 06.11.2020 20:00

Mathematics, 06.11.2020 20:00

Mathematics, 06.11.2020 20:00

Biology, 06.11.2020 20:00

Chemistry, 06.11.2020 20:00

History, 06.11.2020 20:00

Mathematics, 06.11.2020 20:00




English, 06.11.2020 20:00




Mathematics, 06.11.2020 20:00


English, 06.11.2020 20:00


 years ago.
years ago. years
years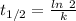
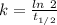
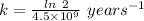
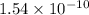 years⁻¹
years⁻¹![[A_t]=[A_0]e^{-kt}](/tpl/images/0407/4769/1ef89.png)
![[A_t]](/tpl/images/0407/4769/5262c.png) is the final concentration= 0.275 mg
is the final concentration= 0.275 mg![[A_0]](/tpl/images/0407/4769/9a686.png) is the initial concentration = 1 mg
is the initial concentration = 1 mg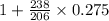 mg = 1.297 mg
mg = 1.297 mg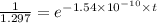
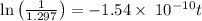
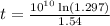
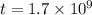 years
years

