
Chemistry, 07.12.2019 04:31 rleiphart1
A) moles of co2 required to inflate 160-l front passenger-side air bag at room temperature and pressure
b) balanced equation for the reaction of nahco3 and ch3cooh to co2
c)mass of nahco3 needed for the reaction (84.0 g/mol)
d) volume of vinegar required (0.833 m acetic acid)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
A) moles of co2 required to inflate 160-l front passenger-side air bag at room temperature and press...
Questions




Mathematics, 20.08.2019 16:20

Biology, 20.08.2019 16:20



Mathematics, 20.08.2019 16:20


Mathematics, 20.08.2019 16:20

Mathematics, 20.08.2019 16:20

Social Studies, 20.08.2019 16:20


Mathematics, 20.08.2019 16:20

Mathematics, 20.08.2019 16:20


Mathematics, 20.08.2019 16:20


Social Studies, 20.08.2019 16:20

English, 20.08.2019 16:20

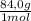 = 549g of NaHCO₃
= 549g of NaHCO₃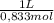 = 7,85L of CH₃COOH
= 7,85L of CH₃COOH

