
Chemistry, 07.12.2019 04:31 maxy7347go
In which solution(s) will a precipitate form? i. 0.00090 g of na2cro4 (molar mass = 162.0 g/mol) is added to 225 ml of 0.00025 m agno3. ksp ag2cro4 =1.1 x 10-12 ii. 0.100l of 0.0015 m mgcl2 and 0.200l of 0.012 m naf. ksp mgf2 = 3.7 x 10-8

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 22.06.2019 20:40
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1
You know the right answer?
In which solution(s) will a precipitate form? i. 0.00090 g of na2cro4 (molar mass = 162.0 g/mol) is...
Questions


English, 29.06.2019 16:20


Health, 29.06.2019 16:20


History, 29.06.2019 16:20


Mathematics, 29.06.2019 16:20



Social Studies, 29.06.2019 16:20



Mathematics, 29.06.2019 16:20

Mathematics, 29.06.2019 16:20


Biology, 29.06.2019 16:20

Computers and Technology, 29.06.2019 16:20

Spanish, 29.06.2019 16:20

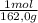 = 5,56x10⁻⁶ moles of Na₂CrO₄≡ moles of CrO₄²⁻.
= 5,56x10⁻⁶ moles of Na₂CrO₄≡ moles of CrO₄²⁻.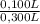 = 0,0005M MgCl₂≡ Mg²⁺
= 0,0005M MgCl₂≡ Mg²⁺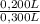 = 0,008M NaF ≡ F⁻
= 0,008M NaF ≡ F⁻

