

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
An atom (not a hydrogen atom) absorbs a photon whose associated wavelength is 300 nm and then immedi...
Questions

Computers and Technology, 27.07.2021 02:00



Computers and Technology, 27.07.2021 02:00

Health, 27.07.2021 02:00





Mathematics, 27.07.2021 02:00





Mathematics, 27.07.2021 02:00



Mathematics, 27.07.2021 02:00

Mathematics, 27.07.2021 02:00

 - 1/λ
- 1/λ )
)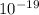 J.
J.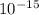 eVs
eVs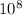 m/s
m/s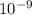 m
m )
) 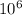 eV = 2.196 eV
eV = 2.196 eV

