
Chemistry, 07.12.2019 06:31 emmarieasimon
Acertain first-order reaction (a→products) has a rate constant of 7.20×10−3 s−1 at 45 ∘c. how many minutes does it take for the concentration of the reactant, [a], to drop to 6.25% of the original concentration?
express your answer with the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
Acertain first-order reaction (a→products) has a rate constant of 7.20×10−3 s−1 at 45 ∘c. how many m...
Questions

Biology, 30.10.2019 18:31

Mathematics, 30.10.2019 18:31

Mathematics, 30.10.2019 18:31

Business, 30.10.2019 18:31



Mathematics, 30.10.2019 18:31

Mathematics, 30.10.2019 18:31

Mathematics, 30.10.2019 18:31

English, 30.10.2019 18:31

Social Studies, 30.10.2019 18:31

Biology, 30.10.2019 18:31

Geography, 30.10.2019 18:31


Chemistry, 30.10.2019 18:31



English, 30.10.2019 18:31


Mathematics, 30.10.2019 18:31

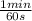 = 6,41 min
= 6,41 min

