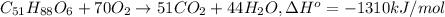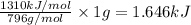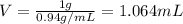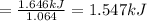Chemistry, 07.12.2019 06:31 school4life110
One possible use for the cooking fat left over after making french fries is to burn it as fuel.
formula = c(51)h(88)o(6)
density = 0.94 g/ml
delta h^degree = - 1310 kj/mol.
write a balanced equation of the combustion of cooking fat. use the data above to calculate the amount of energy released (in kilojoules per milliliter) from the combustion of cooking fat:

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1
You know the right answer?
One possible use for the cooking fat left over after making french fries is to burn it as fuel.
Questions


Biology, 30.04.2021 17:20

Mathematics, 30.04.2021 17:20


Mathematics, 30.04.2021 17:20



Mathematics, 30.04.2021 17:20


English, 30.04.2021 17:20

Chemistry, 30.04.2021 17:20




English, 30.04.2021 17:20


Mathematics, 30.04.2021 17:20

Mathematics, 30.04.2021 17:20