
Chemistry, 01.12.2019 00:31 deaishaajennings123
Calculate the approximate enthalpy change, δhrxn, for the combustion of methane: ch4+2o2→2h2o+co2 δhrxn from a given table: ch4 = 1656 kj/mol o2 = 498 kj/mol h2o = 928 kj/mol co2 = 1598 kj/mol?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3

Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
You know the right answer?
Calculate the approximate enthalpy change, δhrxn, for the combustion of methane: ch4+2o2→2h2o+co2 δ...
Questions


Mathematics, 20.01.2020 02:31


Mathematics, 20.01.2020 02:31


Mathematics, 20.01.2020 02:31






Mathematics, 20.01.2020 02:31



Health, 20.01.2020 02:31


Mathematics, 20.01.2020 02:31

Mathematics, 20.01.2020 02:31

Spanish, 20.01.2020 02:31

History, 20.01.2020 02:31

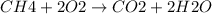
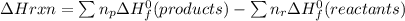
![\Delta Hrxn = [2\Delta H_{f}^{0}(H2O)+1\Delta H_{f}^{0}(CO2)]-[1\Delta H_{f}^{0}(CH4)+ 2\Delta H_{f}^{0}(O2)]](/tpl/images/0397/7957/b0337.png)


