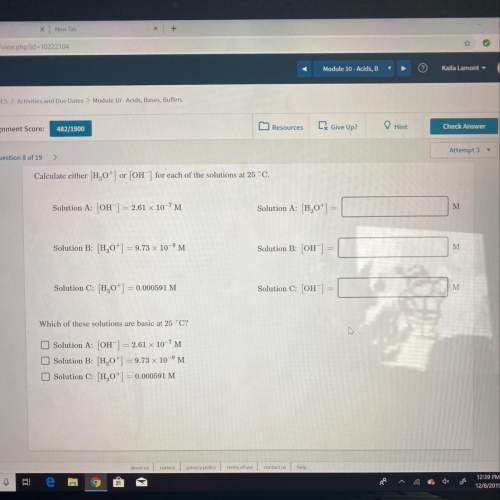
Calculate either h,0") or (oh") for each of the solutions at 25°c.
solution a: (oh) = 2.61 x...

Chemistry, 09.12.2019 01:31 hannahsparks7073
Calculate either h,0") or (oh") for each of the solutions at 25°c.
solution a: (oh) = 2.61 x 10-7m
solution a: 1,0+1 =
solution b: (,0) = 9.73 x 10-'m
solution b: oh
solution c: h,0+1 = 0.000591 m
solution c: [oh] = 0
which of these solutions are basic at 25 °c?
solution a: (oh) = 2.61 x 10-7m
solution b: h,0+) = 9.73 x 10-'m
solution c: h0+] = 0.000591 m

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2
You know the right answer?
Questions

History, 20.04.2020 02:42



English, 20.04.2020 02:42

Biology, 20.04.2020 02:43

History, 20.04.2020 02:43



Mathematics, 20.04.2020 02:43


English, 20.04.2020 02:43

History, 20.04.2020 02:43

Mathematics, 20.04.2020 02:43



Mathematics, 20.04.2020 02:43

Mathematics, 20.04.2020 02:44

Engineering, 20.04.2020 02:44




