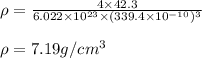Chemistry, 22.08.2019 08:30 domenica19
The atomic radius of metal x is 1.20 × 102 picometers (pm) and a crystal of metal x has a unit cell that is face-centered cubic. calculate the density of metal x? (atomic weight = 42.3 g/mol)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
The atomic radius of metal x is 1.20 × 102 picometers (pm) and a crystal of metal x has a unit cell...
Questions

Chemistry, 25.01.2021 21:10

History, 25.01.2021 21:10

Mathematics, 25.01.2021 21:10


Mathematics, 25.01.2021 21:10

Biology, 25.01.2021 21:10

Spanish, 25.01.2021 21:10

Mathematics, 25.01.2021 21:10



Mathematics, 25.01.2021 21:10


Mathematics, 25.01.2021 21:10




Mathematics, 25.01.2021 21:10



Mathematics, 25.01.2021 21:10

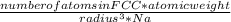 . Substituting the given, the density is 162.69 g/cm3.
. Substituting the given, the density is 162.69 g/cm3. 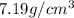
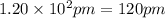
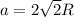
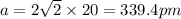
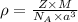
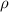 = density
= density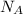 = Avogadro's number =
= Avogadro's number = 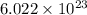
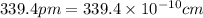 (Conversion factor:
(Conversion factor: 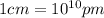 )
)