
Chemistry, 09.12.2019 17:31 makayla4141
X2 + y2 > x2y2 rate = k[x2]a reaction and its experimentally determined rate law are represented above. a chemist proposes two different possible mechanisms for the reaction, which are given below. mechanism 1 mechanism 2x2 > 2x (slow) x2 > 2x (slow)x + y2 > xy2 (fast) x + y2 > xy + y (fast)x + xy2 > x2y2 (fast) x + xy > x2y (fast)x2y + y > x2y2 (fast)based on the information above, which of the following is true? both mechanism 1 and 2 are consistent with the rate law. only mechanism 2 is consistent with the rate law. neither mechanism 1 nor mechanism 2 is consistent with the rate law. only mechanism 1 is consistent with the rate law.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3
You know the right answer?
X2 + y2 > x2y2 rate = k[x2]a reaction and its experimentally determined rate law are represented...
Questions

Advanced Placement (AP), 31.10.2020 04:50

Spanish, 31.10.2020 04:50

Social Studies, 31.10.2020 04:50



English, 31.10.2020 04:50


Mathematics, 31.10.2020 04:50

Arts, 31.10.2020 04:50

Social Studies, 31.10.2020 04:50

Mathematics, 31.10.2020 04:50

Mathematics, 31.10.2020 04:50




Social Studies, 31.10.2020 04:50

Mathematics, 31.10.2020 04:50

Arts, 31.10.2020 04:50


Business, 31.10.2020 04:50

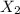 ⇒
⇒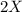 is the slowest step or RATE DETERMINING STEP .
is the slowest step or RATE DETERMINING STEP .![[X_2]](/tpl/images/0410/1202/df4e1.png) .
.

