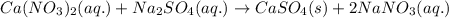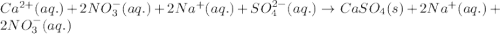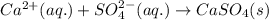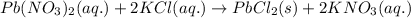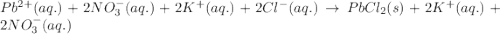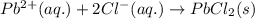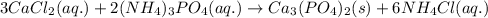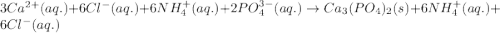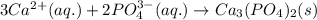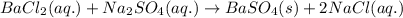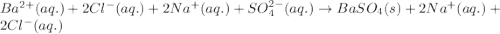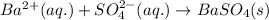Chemistry, 09.12.2019 17:31 jbehrens6538
Write the net ionic equation to show the formation of a solid (insoluble salt) when the following solutions are mixed. write noreaction if there is no precipitate. express your answer as a chemical equation. identify all of the phases in your answer. enter noreaction if no precipitate is formed.1. ca(no3)2(aq) + na2so4(aq)2. kcl(aq) + pb(no3)2(aq)3. cacl2(aq) + (nh4)3(po4)(aq)4. na2so4(aq) + bacl2(aq)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

You know the right answer?
Write the net ionic equation to show the formation of a solid (insoluble salt) when the following so...
Questions

History, 26.08.2020 19:01

English, 26.08.2020 19:01

Mathematics, 26.08.2020 19:01

English, 26.08.2020 19:01

Mathematics, 26.08.2020 19:01

Health, 26.08.2020 19:01

Physics, 26.08.2020 19:01


Advanced Placement (AP), 26.08.2020 19:01

English, 26.08.2020 19:01

Biology, 26.08.2020 19:01





History, 26.08.2020 19:01

Mathematics, 26.08.2020 19:01


Biology, 26.08.2020 19:01

History, 26.08.2020 19:01